N95 Respirator Shortage Prompts Additional Guidance from OSHA
OSHA's newest interim guidance issued on April 3 concerns all American workers exposed to respiratory hazards along with healthcare personnel (HCP) on the front lines of the COVID-19 outbreak.
The memo follows a March 11 memorandum that allows healthcare employers to temporarily switch from a quantitative fit testing method to a qualitative testing method to preserve the integrity of N95 respirators.
"Due to the impact on workplace conditions caused by limited supplies of N95 filtering facepiece respirators (FFRs), all employers should reassess their engineering controls, work practices, and administrative controls to identify any changes they can make to decrease the need for N95 respirators," the memorandum states.
Hospital systems across the country have expressed the dire need for personal protective equipment (PPE) since the initial outbreak healthcare providers saw a drastic rise in the number of patients.
Some companies have converted their manufacturing plants to produce face masks, while others have set out to improve mask decontamination methods.
Retailers such as Home Depot that sell N95 respirators have cut off sales to the public to ensure frontline workers receive supplies first.
Federal, state and local agencies also have focused efforts on PPE hoarding schemes related to the COVID-19 pandemic.
Extending and Reusing
In accordance with the temporary guidance, OSHA is permitting extended use or reuse of respirators as long as the PPE "maintains structural and functional integrity and the filter material is not physically damaged, soiled, or contaminated (e.g., with blood, oil, paint)."
All employers must revise their respiratory protection programs (RPPs) to include the circumstances under which the respirator is contaminated and not available for "extended use or reuse."
Because of contact transmission hazards, OSHA recommends extended use over reuse due to the risk of donning/doffing. Employers also need to be cognizant of how workers are storing FFRs between periods of reuse.
The memorandum recommends increasing the use of wet methods or portable local exhaust systems or even moving tasks outdoors. Non-essential operations could also be suspended for the duration of the pandemic.
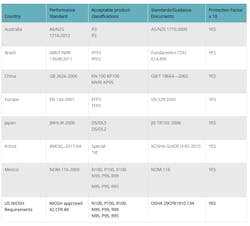
If all of these conditions have been met and N95 respirator use still is required, the agency pointed to National Institute for Occupational Safety and Health's (NIOSH's) approved list of comparable respirators:
Centers for Disease Control and Prevention (CDC)
Employers must show they made a "good faith effort" to obtain N95 respirators or similar before reuse or use of expired N95 FFRs is permitted.
A list of NIOSH-certified expired N95 FFR is available here. Company officials are required to notify workers if the PPE they are using is past its recommended shelf life.
In addition, all employers must keep track of inventory to ensure that products purchased are not mixed with expired respirators.
"Employers should visually inspect, or ensure that workers visually inspect, the N95 FFRs to determine if the structural and functional integrity of the respirator has been compromised," the memorandum states. "Over time, components such as the straps, nose bridge, and nose foam material may degrade, which can affect the quality of the fit and seal. Where an employer has expired N95s available from their own stored cache (i.e., not from the U.S. Strategic National Stockpile), the employer should seek assistance from the respirator manufacturer or independent lab regarding testing of those stored respirators prior to use."
Additional Guidance for Healthcare Employers
While the enforcement of proper respiratory protection for the majority of employers allows use of expired N95s, measures for healthcare workers remain more stringent.
Expired PPE should not be used by those who "perform surgical procedures on patients infected with, or potentially infected with, SARS-CoV-2, or perform or are present for procedures expected to generate aerosols or procedures where respiratory secretions are likely to be poorly controlled (e.g., cardiopulmonary resuscitation, intubation, extubation, bronchoscopy, nebulizer therapy, sputum induction)."
The agency points to previous guidance released by the Centers for Disease Control and Prevention (CDC), saying the use of N95 respirators needs to be prioritized by activity type.
"When HCP perform or are present for aerosol-generating procedures or procedures where respiratory secretions are likely to be poorly controlled, use respirators (including N95 FFRs; other FFRs; non-disposable, elastomeric respirators; and PAPRs) that are still within their manufacturer’s recommended shelf life, if available, before using respirators that are before using respirators that are beyond their manufacturer’s recommended shelf life," the memorandum says.
Enforcement
OSHA also addressed compliance in its latest memorandum, saying it will practice discretion when issuing citations in violation or it 29 CFR § 1910.134(d) respiratory protection standard.
The agency noted the following causes for consideration:
- The employer has made a good faith effort to obtain other alternative filtering facepiece respirators, reusable elastomeric respirators, or PAPRs appropriate to protect workers;
- The employer has monitored their supply of N95s and prioritized their use according to CDC guidance.
- Surgical masks and eye protection (e.g., face shields, goggles) were provided as an interim measure to protect against splashes and large droplets (note: surgical masks are not respirators and do not provide protection against aerosol-generating procedures); and
- Other feasible measures, such as using partitions, restricting access, cohorting patients (healthcare), or using other engineering controls, work practices, or administrative controls that reduce the need for respiratory protection, were implemented to protect employees.
"Where the above efforts are absent and respiratory protection use is required, or voluntary use is permitted, and an employer fails to comply with fit testing, maintenance, care, and training requirements, cite the applicable provision(s) of 29 CFR § 1910.134 and/or other applicable expanded health standards as serious violations," OSHA concluded.
The complete enforcement memo is available on OSHA's website.
About the Author

Stefanie Valentic
Stefanie Valentic was formerly managing editor of EHS Today, and is currently editorial director of Waste360.